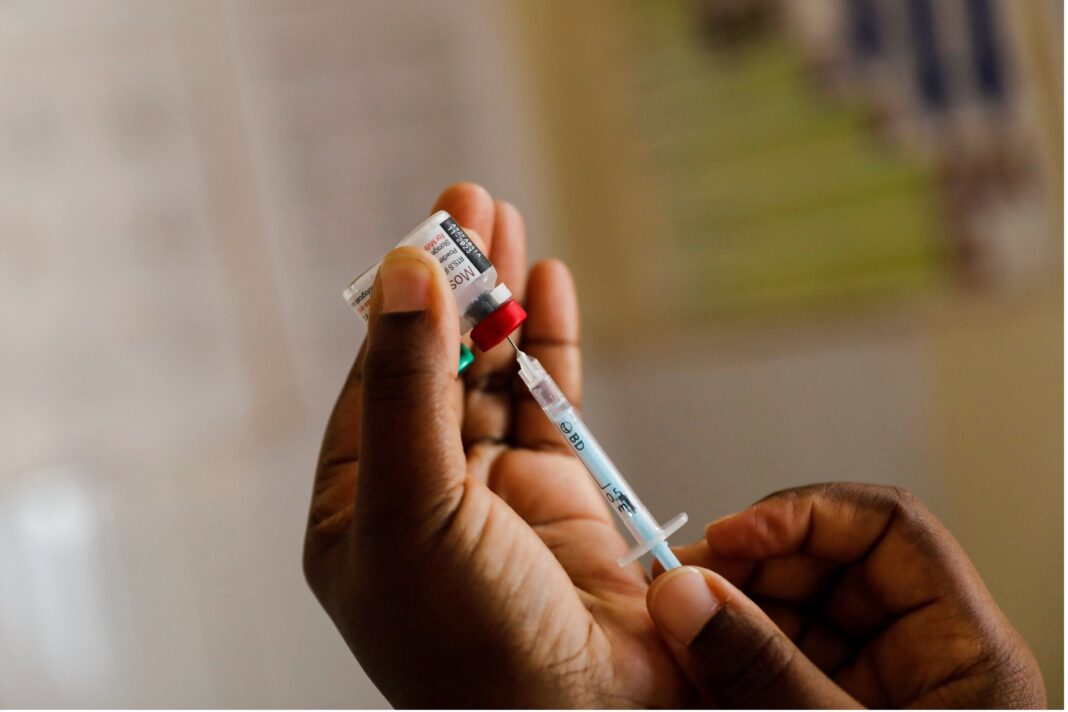
Ghana has become the first country to give approval to a malaria vaccine developed by the Oxford University.
The African country at the moment is expanding its malaria vaccination drive and the latest approval would help shore that up.
Ghana is already using the first malaria vaccine, Mosquirix from British drugmaker GSK which was last year endorsed by the World Health Organization (WHO).
The Oxford vaccine is now approved for children aged 5 months to 36 months, the age group at highest risk of death from malaria.
Serum Institute of India is to produce up to 200 million doses of the vaccine annually.
This is the first time a major vaccine like this is getting approval in an African country, before rich nations.
Oxford scientist Adrian Hill said said Hill it was unusual that a regulatory authority in Africa had reviewed the data on the vaccine quicker than the WHO.
“Particularly since COVID, African regulators have been taking a much more proactive stance, they’ve been saying…we don’t want to be last in the queue.”
In contrast, GSK has committed to produce up to 15 million doses of Mosquirix every year through 2028, which is well under the roughly 100 million doses a year of the four-dose vaccine the WHO says is needed long-term to cover around 25 million children.
Malaria kills over 600,000 each year, most of them children in Africa.
WHO gives approval for mass use of malaria vaccine in Africa
Source: Africafeeds.com