Britain on Wednesday said it had approved the Pfizer-BioNTech vaccine for use and that it will be rolled out from next week.
“The Government has today accepted the recommendation from the independent Medicines and Healthcare products Regulatory Agency (MHRA) to approve Pfizer-BioNTech’s COVID-19 vaccine for use,” the government said.
“The vaccine will be made available across the UK from next week.”
The move makes Britain one of the first countries to begin the process of vaccinating its population as it tries to the COVID-19 outbreak.
Prime Minister Boris Johnson said COVID-19 vaccines will “allow us to reclaim our lives.”
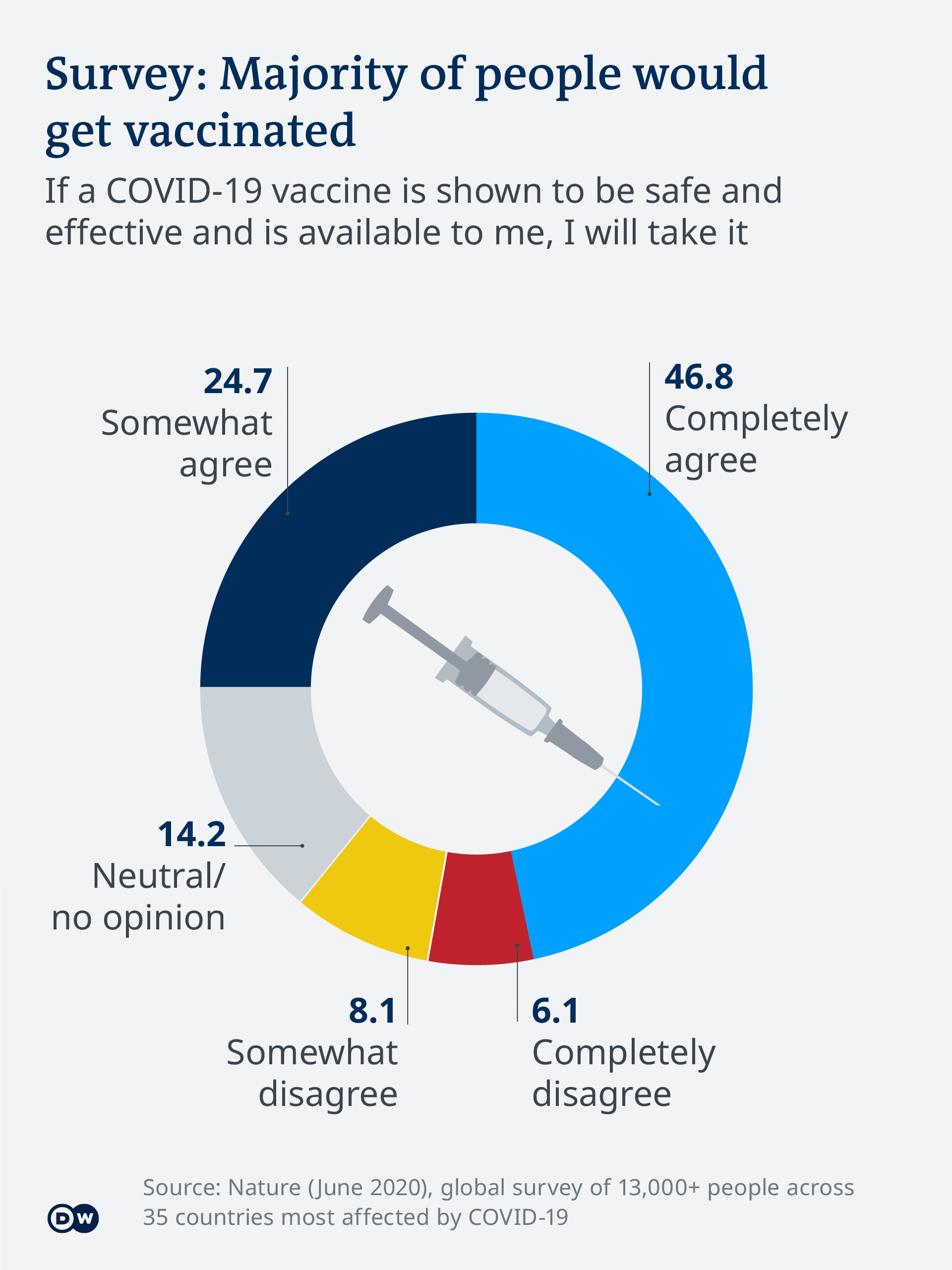
Vaccinations could start next week
Other countries aren’t far behind: The US and the European Union also are vetting the Pfizer shot along with a similar vaccine made by competitor Moderna Inc. British regulators also are considering another shot made by AstraZeneca and Oxford University.
British media have reported that hospitals in England have been told to get ready to start doing vaccinations for medical workers as early as next week, AP reports.
Pfizer said it would immediately begin shipping limited supplies to the UK — and has been gearing up for even wider distribution if given a similar nod by the US Food and Drug Administration, a decision expected as early as next week.
But doses everywhere are scarce, and initial supplies will be rationed until more is manufactured in the first several months of next year.
Pfizer CEO Albert Bourla called the UK decision ”a historic moment.”
”We are focusing on moving with the same level of urgency to safely supply a high-quality vaccine around the world,” Bourla said in a statement. “This authorization is a goal we have been working toward since we first declared that science will win, and we applaud the MHRA for their ability to conduct a careful assessment and take timely action to help protect the people of the UK,” he said.
‘No corners cut’
Amid criticism of the vaccine’s approval process, the MHRA said that no corners had been cut in developing and testing the vaccine. “Everybody can be confident that no corners whatsoever have been cut,” MHRA chief executive June Raine.
“The public deserve nothing less,” she added, stressing that her agency’s certification process was no different to counterparts in the US and EU.
“Our expert scientists and clinicians have worked round the clock, carefully, methodically, poring over tables and analyses and graphs on every single piece of data, hundreds, over a thousand pages of data and, absolutely critically, analyzing the pre-clinical evidence, the clinical trials, the manufacturing and quality controls, and then down to the final sampling,” she said.
A ‘problematic’ approval process
The comments follow criticisms made by the European Medicines Agency, which is in charge of approving vaccines for the EU, said its longer procedure to approve vaccines was more appropriate as it was based on more evidence adn required more checks than the emergency procedure chosen by Britain. EU lawmakers were also critical of the approval.
“I consider this decision to be problematic and recommend that EU Member States do not repeat the process in the same way,” said Peter Liese, an EU lawmaker who is a member of German Chancellor Angela Merkel’s party.
“A few weeks of thorough examination by the European Medicines Agency is better than a hasty emergency marketing authorization of a vaccine,” said Liese.
“There is an obvious global race to get the vaccine on the market as fast as possible,” said Tiemo Wolken, an EU lawmaker. “However, I do believe that it is better to take the time and make sure that the quality, effectiveness and safety is guaranteed and matches our EU standards.”
Hard winter still in store
While the UK has ordered enough Pfizer vaccine for 20 million people, it’s not clear how many will arrive by year’s end. Adding to the distribution challenges, the Pfizer vaccine must be stored at ultra-cold temperatures. Two doses three weeks apart are required for protection.
The UK government says frontline health care workers and nursing home residents will be first in line to get vaccinated, followed by older adults.
But Prime Minister Boris Johnson has warned ”we must first navigate a hard winter” of restrictions to try to curb the virus until there’s enough vaccine to go around for everyone who wants to be vaccinated.
Every country has different rules for determining when an experimental vaccine is safe and effective enough to use. Intense political pressure to be the first to roll out a rigorously scientifically tested shot colored the race in the United States and Britain, even as researchers pledged to cut no corners. In contrast, China and Russia have offered different vaccinations to their citizens ahead of late-stage testing.
But experts caution that a vaccine cleared for emergency use is still experimental and the final testing must be completed. Still to be determined is whether the Pfizer-BioNTech shots protect against people spreading the coronavirus without showing symptoms.
Another question is how long protection lasts. The vaccine also has been tested in only a small number of children, none younger than 12, and there’s no information on its effects in pregnant women.
Kenya says it will reach out to Russia for its Covid-19 vaccine
Source: DW (Reuters, AFP, AP)